
Insparya
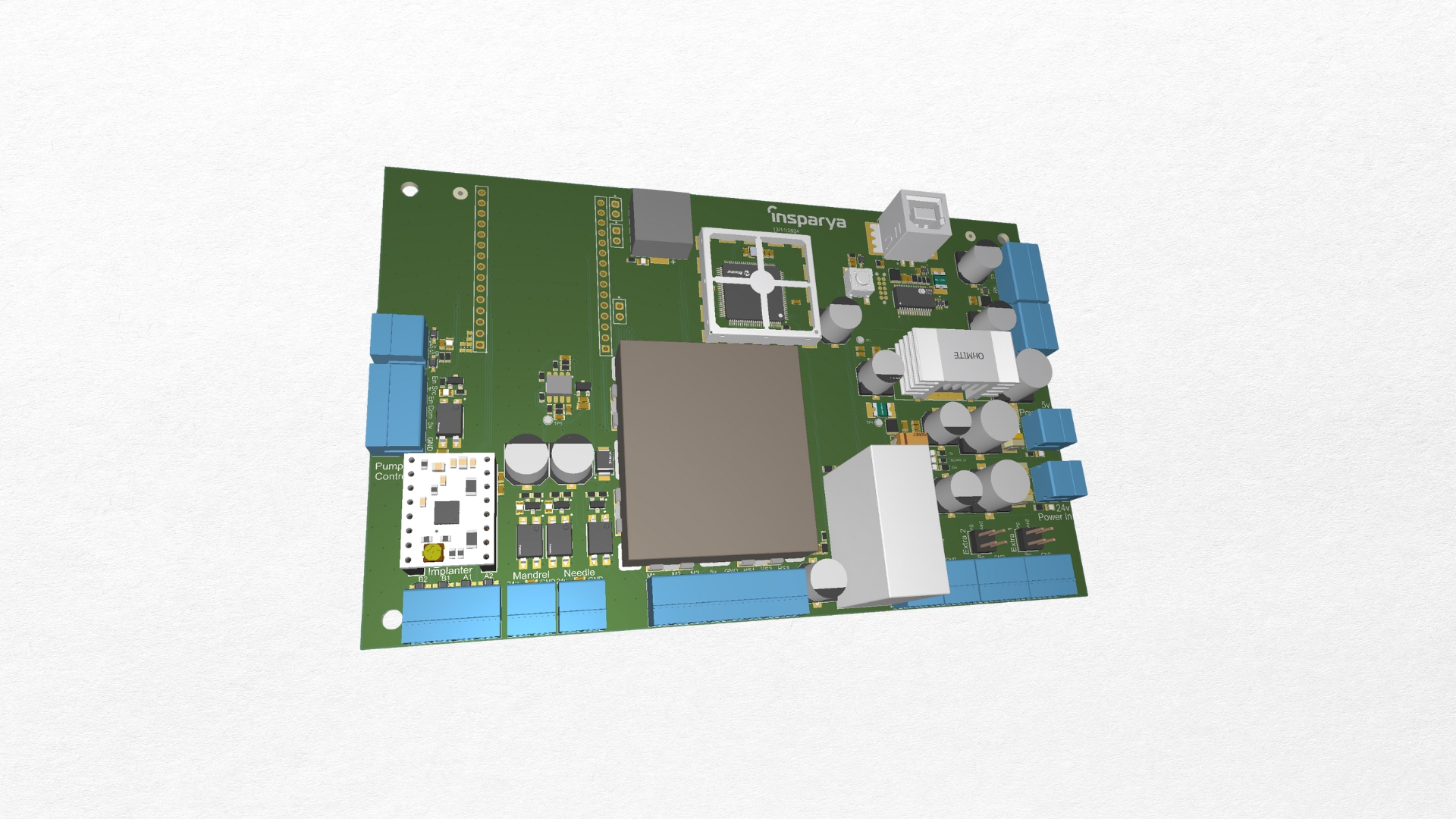
Insparya had developed a capillary transplant machine, but the product failed to meet compliance requirements during certification. Detus was brought in to resolve these issues, focusing on the redesign and validation of the hardware. We engineered a new PCB, carried out in-house testing, and evaluated the machine’s critical points to ensure every parameter aligned with the necessary standards.
The main challenge was taking a product that was already in its final stage and addressing fundamental compliance failures without disrupting its overall functionality. This required rebuilding the PCB to meet regulatory requirements while maintaining the machine’s intended performance. Identifying and correcting critical weaknesses through rigorous in-house testing was essential to ensure the device could pass certification.
With the new PCB and validated hardware, the capillary transplant machine successfully met compliance standards and achieved certification. This allowed Insparya to move forward with confidence, bringing their product to market and strengthening their position as a leading innovator.
